Abstract
Background In older patients with NPM1 mutated acute myeloid leukemia (AML), venetoclax combined with azacitidine or low dose cytarabine (LDAC) is associated with CR/CRi rates of 67% and 79% respectively (DiNardo, NEJM 2020; Wei, Blood 2020). While molecular monitoring of measurable residual disease (MRD) in patients with NPM1 mutated AML treated with intensive chemotherapy is strongly prognostic and can be used to guide therapeutic decisions (Ivey, NEJM 2016; Balsat, JCO 2017), its value in those receiving venetoclax combinations is unknown. Flow cytometric MRD is predictive in unselected patients receiving venetoclax and azacitidine but appears less discriminative in the NPM1 mutated subgroup (Pratz, JCO 2021).
Methods Patients were identified from real-world cohorts of AML treated with venetoclax and LDAC or hypomethylating agents (HMA) in the United Kingdom, and Melbourne, Australia. Inclusion criteria were i) NPM1 mutation, ii) frontline treatment with venetoclax combination, iii) achievement of complete remission with or without count recovery as assessed by the treating haematologist and iv) at least one bone marrow MRD assessment in the first 6 months of therapy. MRD was performed at central reference laboratories using established RT-qPCR assays, with NPM1 copy numbers reported relative to 100 copies of ABL. Overall survival (OS) and event-free survival (EFS) were measured from the day of starting therapy and defined as per ELN 2022 guidelines.
Results Fifty-four patients were identified with a median age of 72.7 years and 52% female. Performance status was 0 or 1 in 46 patients (87%). AML was de novo in 44 (83%) and the karyotype was intermediate risk in 49 (92%). A FLT3-ITD was present in 16 patients (30%) and FLT3-TKD in 8 (15%). Next-generation sequencing results were available in 42 patients in whom a DNMT3A mutation was detected in 14 (33%), IDH2 in 9 (21%) and IDH1 in 4 (10%). ELN 2017 risk was favourable in 45 (85%), intermediate in 6 and adverse in 2.
Twenty-seven patients received venetoclax in combination with azacitidine (50%), 2 with decitabine (4%) and 25 with LDAC (46%). Within the first 6 months of therapy, patients had a median of 2 bone marrow response assessments (range 1-4). The NPM1 copy number reduced over time: in the first 3 months the median was 1.0 (IQR 0.03-3.82) and in the second 3 months it was 0.0 (IQR 0-0.05). Twenty-five patients (46%) achieved bone marrow MRD negativity after a median of 97 days (range 30 to 157). In the remaining patients, the best reduction achieved (compared to the diagnostic sample) was ≥3 log10 in 16 (30%) and <3 log10 in 12 (23%). There was no difference in depth of response between those treated with HMAs and LDAC, with MRD negativity achieved in 45% and 48% and at least a 3 log10 reduction in 76% and 79% respectively.
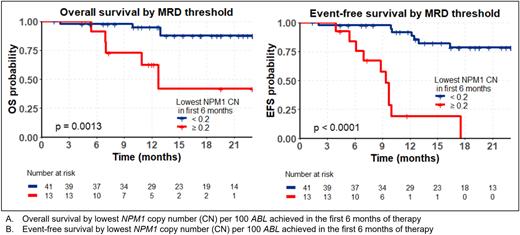
With a median follow-up time of 17.4 months, OS for all patients at 18 months was 79% (95% confidence interval [95CI] 67-92) while EFS was 61% (95CI 47-78). The deepest MRD reduction within the first 6 months was strongly associated with both OS and EFS, which at 18 months were 95% and 85% if MRD negativity was reached, 73% and 60% with a positive result but ≥3 log10 reduction and 39% and 0% if <3 log10 reduction (p=0.0012 for OS and p<0.0001 for EFS). Maximally selected rank statistics were used to identify a threshold NPM1 copy number of 0.2 per 100 ABL which best predicted EFS, which was also strongly associated with OS (Figures A and B). The NPM1 threshold of 0.2 copies corresponded to a >3 log10 reduction in the majority of patients. On multivariate cox regression including age, performance status, de novo vs secondary AML and FLT3-ITD, only the copy number threshold predicted outcomes, with a hazard ratio of 16.8 (95CI 2.97-95.1 p=0.001) for OS and 19.8 (95CI 5.3-73.4, p=<0.001) for EFS in those not reaching <0.2 copies of NPM1 per 100 ABL in the first 6 months of therapy.
Conclusion In patients with NPM1 mutated AML attaining complete remission with venetoclax combination therapies, achievement of an NPM1 copy number of <0.2 per 100 ABL, or a ≥3 log10 reduction from the diagnostic result, in the bone marrow within the first 6 months of therapy was strongly associated with excellent clinical outcomes.
Disclosures
Tiong:Servier: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Amgen: Speakers Bureau. Latif:Jazz: Honoraria; Novartis: Honoraria; Kite: Honoraria; Abbvie: Honoraria; Astellas: Honoraria; Takeda UK: Honoraria. Fong:Otsuka: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Hogan:Abbvie: Honoraria, Other: support for conference attendance. Khan:Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; TC BioPharm: Membership on an entity's Board of Directors or advisory committees; Astellas: Speakers Bureau; Abbvie: Honoraria; Gilead: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Krishnamurthy:Astellas: Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria. Marshall:Abbvie: Honoraria, Other: Advisory board. Murthy:Abbvie: Honoraria; Jazz: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: NA. Nagumantry:Janssen: Honoraria; Alexion: Honoraria. Wei:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Employee of the Walter and Eliza Hall Institute and is eligible for a fraction of the royalty stream related to Venetoclax, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dillon:Shattuck Labs: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis AG: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AvenCell Europe GmbH: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma Inc.: Consultancy, Honoraria, Speakers Bureau; Amgen Inc.: Research Funding; AbbVie Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal